(part of Steps Toward Molecular Manufacturing)
- the zeolite-effect:
As the goal is to build machine components from the
MBBs, one would like to obtain solid materials having a stiffness
similar to proteins or wood. These are quite densely packed materials
containing little empty space. It came to me as an unexpected surprise
when it turned out that building lattices with MBBs usually generates
large empty cavities. This phenomenon seems to be very difficult to
avoid. The problem is illustrated symbolically in
figure 3. The very
process of link-formation introduces a few atoms between the cores of
the MBBs. Thus the covalent link starts to act as a spacer which keeps
the skeletons separated at a certain distance. If one follows around
in a loop to build lattice-like structures, one finds that the loop
suddenly encloses a lot of unreachable volume. The effect is much
worse in actual 3D space than in the 2D caricature shown in figure
3. This phenomenon invariably leads to the accidental formation of
zeolite-like structures containing large internal caverns.
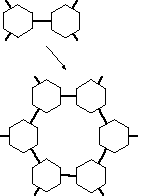
FIGURE 3: Chemical bonds acting as spacers are generating large cavities in a
lattice.
This empty space does not contribute to the structural
stiffness of the material, being filled with solvent molecules, which
of course are too fluid to achieve significant mechanical strength. It
would be very desirable to bring the cores of the MBBs together much
closer, so that their mutual vdW-interactions are able to contribute
to the material stiffness. This is the case in the interior of
proteins, where most of the stability comes only from
weaker-than-covalent interactions. But then on the other hand, the
internal design of proteins does not adhere very much to well
organized rasters, which renders their design much more difficult. A
strategy that might be pursued to reduce the spacer-effect is
illustrated symbolically in figure 4.
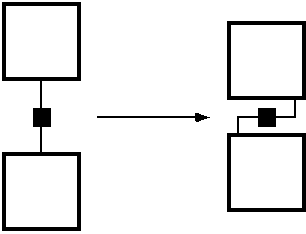
FIGURE 4: Reducing the adverse separation effect of the spacers.
The idea is to fold back the link-chemistry
onto the surface of the MBBs, so that the links do not stand off like
the spines of a hedgehog. An actual implementation might look like
figure 5.
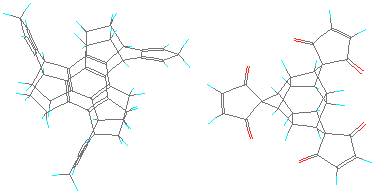
FIGURE 5: A pair of MBBs with three-fold connectivity. The two components
trindan-diene
and iceane-tridiph
can be retrieved for viewing in a molecular modelling program.
Note that this proposed design also
incorporates the separation of the link-polarities to circumvent the
storage problem alluded to above. That is why there are two distinct
types of MBBs here. Only the one on the left side bends back the
link-functionalities somewhat. This example demonstrates the new
problem with this approach. To bend back the links, it is necessary to
introduce more angles in the skeleton, which have to be rigid to avoid
floppy structures. This leads to polycyclic structures which are
increasingly difficult to synthesize (without already having access to
molecular manufacturing methods :-).
Surprisingly, a tendency in current research seems
to have turned the cavity problem into a virtue. Design of zeolites
has become very popular, because of the hope that the large internal
surface areas might be used for catalytic or separational
purposes. But the zeolite designers are encountering a recurring
problem which is again related to the emptiness of the very cavities
they would like to create. Very often not one lattice is generated,
but several, which are independent but mutually interlocked, and so
all of the space is filled out nevertheless. An instructive example
can be found in [Erm88]. This can
be avoided by the following trick. During the growth of the zeolite
crystals, one has to provide template molecules which are able to
claim the cavity-space, to prevent the formation of the
interpenetrating lattices. One of the most successful attempts and a
concise description of the zeolite phenomenon can be found in [HosRob90]. But the issues involved
in getting useful zeolite designs to work, add an additional twist to
the already significant challenge of designing good MBBs.
It seems as if the real challenge in
constructing designer solids lies in achieving dense packing. Airy
zeolite-like structures have been prepared several times, accidentally
and deliberately. But the systematic elimination of the empty spaces
is the difficult task. This situation is reminiscent of the challenges
in the protein engineering community, where the first step of getting
artificial proteins to fold up into roughly the right way, including
secondary structures, seems to have been easier than commonly
imagined, but the last step of getting the amino acid side chains to
organize properly in the interior to obtain really good tight packing,
seems to be unexpectedly hard to accomplish
[Ric92].
Navigational convenience for "Steps Toward Molecular Manufacturing":
[Back to Start]
[Previous Page: Almost No Skeleton]
[Next Page: Research Status]
last updated Oct. 5 1996
e-mail: kr@n-a-n-o.com
[Back to kr's Home Page]
